Help - agent approval: examples - products
This example shows the entries for two different types of Virkon disinfectant – tablet and powder form. If you are logged in, you can click the heading to view the online data sheet and the SDS links to view the safety data sheet from the vendor.
Virkon Tablets
In the case of virkon tablets, a significant amount of information is presented in the SDS, detailing the hazardous nature of the overall product and it lists the constituent parts and their hazards.
- the product needs to be added as a mixture, as it has known constituent parts, rather than as a single 'chemcal'.
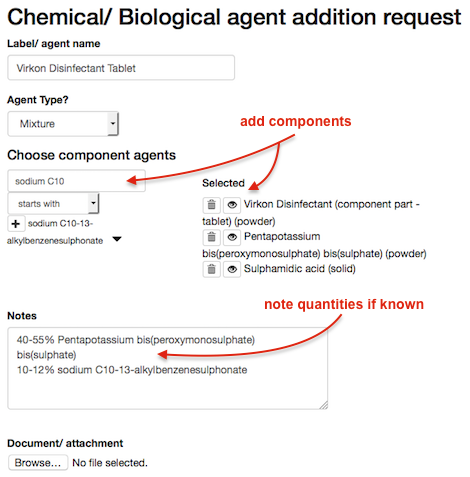
We need to show that the mixture has hazard information, as well as adding the constituent parts to the mixture, so we create a new component part agent.
- request a new chemical agent record for Virkon Disinfectant (component part) - this allows the hazard information for the overall product (listed in section 2) to be included in one component of the mixture record
- when the component part is approved, create the mixture request:
- add our newly approved Virkon Disinfectant (component part)
- add the other constituent parts with their corresponding hazard statements
- in this case, some of the constituent parts were not listed, so these had to be requested/ approved separately
- where the quantity of the component is known, typically as a percentage, this information can be added to the notes of the mixture request
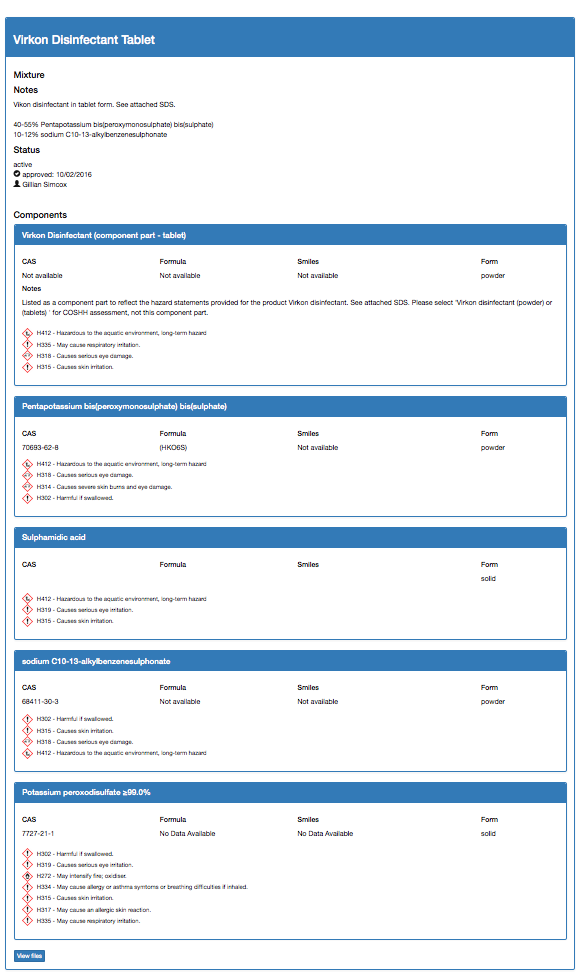
Once approved, the data sheet for Virkon Disinfectant Tablet, with its constituent parts, will look like this:
Virkon Powder
Addition of Virkon disinfectant powder to the system would be carried out in a similar fashion to the tablet - via a mixture agent request.
- the product needs to be added as a mixture, as it has known constituent parts, rather than as a single 'chemcal'.
The powder lists similar constituent parts to the tablet, but note that the hazardous properties listed for the overall powder product varies slightly to that of the tablet form. To account for this, we need to create a new agent request (chemical) for Virkon disinfectant (powder component part) as the component we created for the tablet is different. This can then be used in our new mixture request. .
- request a new chemical agent record for Virkon Disinfectant (powder component part) - this allows the hazard information for the overall product (listed in section 2) to be included in one component of the mixture record
- when the component part is approved, create the mixture request:
- add our newly approved Virkon Disinfectant (powder component part).
- add the other constituent parts with their corresponding hazard statements - any new agents requested when creating the Virkon tablet will already be available.
- where the quantity of the component is known, typically as a percentage, this information can be added to the notes of the mixture request.
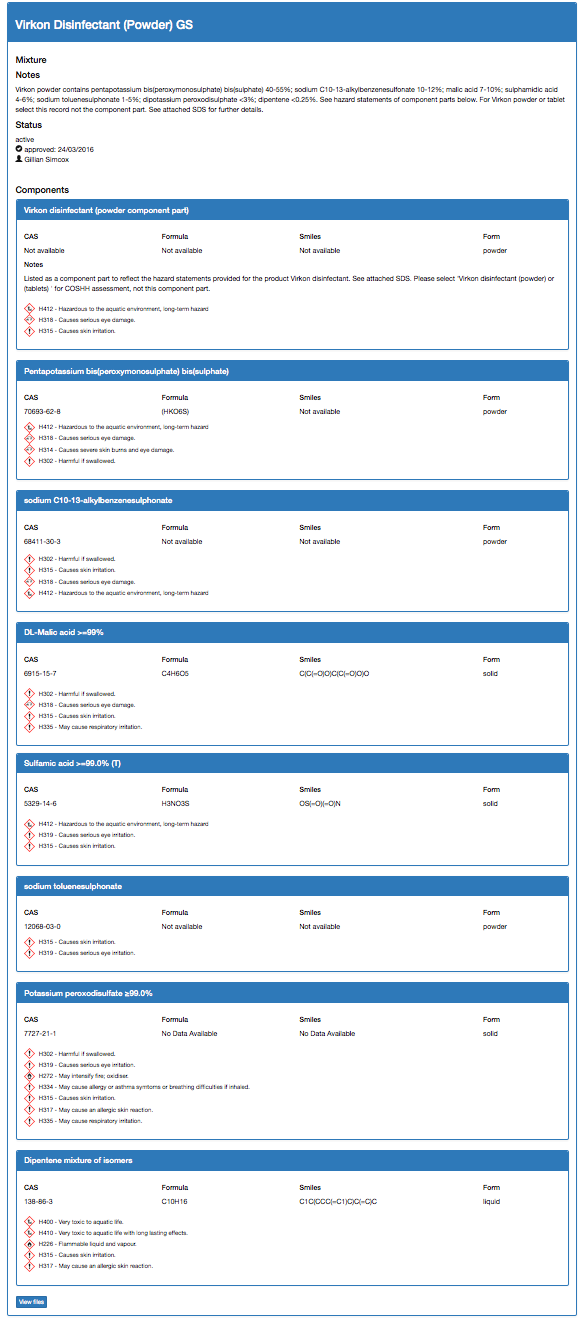
Once approved, the data sheet for Virkon powder will look like this:
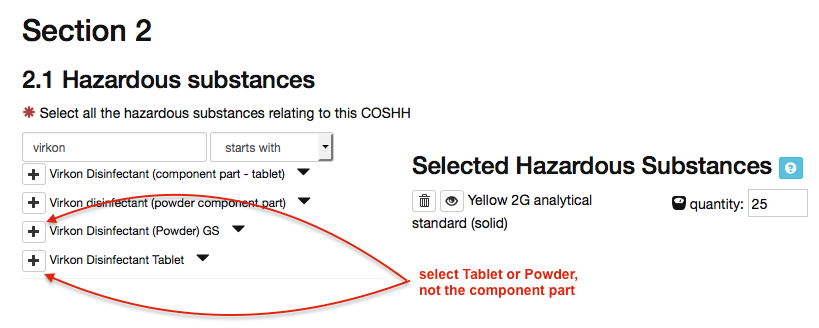
When completing a COSHH form that includes Virkon, if you are working with powdered virkon then select ‘Virkon Disinfectant (Powder) GS’; when working with the tablet form select ‘Virkon Disinfectant Tablet’:
also see: agent examples | agent search | agent request | agent approval